New study suggests ‘unusual’ side effect of weight-loss drugs becoming more common
While weight loss drugs and jabs have become popular, there are some side effects that some people are less aware of that are seemingly becoming more common.
GLP-1 medications such as Wegovy, Zepbound and Saxenda work to suppress appetite and make patients feel full for longer, helping them shed the pounds.
Some people also use Ozempic and Mounjaro, though these are only approved by the FDA to treat type 2 diabetes, and not for weight loss.
Like any medication, there can be some side effects, the most common being loss of appetite, diarrhea, vomiting and nausea.
Then there are the more out-there ones some users have reported, claiming the medication has impacted their penis or vulva.
Now, new research has indicated GLP-1 drugs could be causing a side effect that is disrupting a key procedure often important to medical professionals.

A group of researchers have highlighted their concerning findings (Getty Stock Image)
Researchers from Alliance Medical, a European imaging service provider, found that these medications may be throwing off PET-CT medical imaging scans, which combines a PET scan with a CT scan.
This could ultimately interfere with a doctor’s ability to recognize a person is suffering or could suffer from a deadly disease. It could also lead to misdiagnoses or delay of treatment.
In normal circumstances, according to the Cleveland Clinic, with an FDF PET scan, a patient gets an IV injection of a radiotracer called fluorodeoxyglucose (FDG).
The diseased cells in the patient’s body absorb more of the radiotracer than healthy ones do, and the PET scanner detects these areas.
However, according to the researchers from Alliance Medical, they found several abnormal patterns of FDG uptake in patients taking GLP-1 drugs.
As a result, these abnormal patterns could be misinterpreted by doctors if a patient’s medication history is not considered.
Lead author Dr. Peter Strouhal, Medical Director at Alliance Medical, issued a statement on the researchers’ findings.
Researchers highlighted the issues those on GLP-1 could cause if they have a CT scan (Getty Stock Image)
He said: “We noticed unusual uptake in one of our patients on a GLP-1 agonist, which prompted a wider review across our network.
“We found that these altered patterns are increasingly common, yet there is currently no national or international guidance in the UK addressing this emerging issue.
“Recognizing the characteristic uptake associated with GLP-1 agonists helps avoid unnecessary anxiety and interventions, ensuring patients receive the right care, at the right time, without detours or doubt.”
UNILAD has contacted Novo Nordisk and Eli Lilly for comment.
Featured Image Credit: Getty Stock Image

A study has revealed an alternative and more effective weight loss strategy compared to Ozempic-like drugs, but it comes with a catch.
GLP-1 medications like Ozempic, Mounjaro and Wegovy are typically prescribed to treat Type-2 diabetes, helping patients to manage their blood sugar levels.
While it isn’t technically licensed as a weight loss treatment by the FDA, its active ingredient, semaglutide, works to suppress appetite and has helped hundreds of thousands of people shed more than a few stubborn pounds.
Dozens of Hollywood’s famous faces are looking noticeably slimmer as a result of the medication, though GLP-1 can come with a raft of unfavourable side effects, from nausea, diarrhoea and abdominal pain to sagging skin, a change in libido and even teeth.
Now, a group of researchers have discovered there could be an alternative treatment plan that’s five times more effective at losing weight compared to the jabs: and that’s bariatric or metabolic surgery.
While weight loss surgery is considered a common and safe procedure, as it works to make tweaks to the digestive system, there can be some pretty nasty side effects to be mindful of before jumping under the knife.
Semaglutide pens have been known to help patients shift a lot of weight (Getty Stock Image)
According to the Mayo Clinic, bariatric surgery is considered only in cases where patients have tried to lose weight by improving their diet and exercise habits or who have serious health problems as a result of their weight, like high blood pressure, Type-2 diabetes, sleep apnea or nonalcoholic fatty liver disease, amongst others.
As well as being notoriously expensive, there are several short-term risks to factor in, like excessive bleeding, infection, a reaction to anaesthesia, breathing problems and/or leaks in the gastrointestinal system.
According to the NHS, blood clots can form in the lower leg (deep vein thrombosis) after surgery because of how the blood flows and clots after surgery, though it can be managed with medications.
A stomach leak may also occur in the aftermath of a gastric bypass or sleeve gastrectomy, during which food could leak out of the abdomen and pose a serious infection that requires antibiotic treatment.
At the other end of the spectrum, patients might experience a blocked gut where food can become ‘stuck,’ though this too can easily be mitigated by cutting food into smaller bites and drinking during meals.
Post-surgery, patients are advised that their smaller stomachs might take some getting used to as well.
The surgery involves reducing the size of the stomach (Getty Stock Image)
Though most of these are temporary problems, the procedure also poses a lengthy list of long-term risks.
The Mayo Clinic states bowel obstruction, dumping syndrome – involving diarrhoea, lightheadedness, nausea and vomiting – gallstones, hernias, hypoglycaemia (low blood sugar), ulcers, acid reflux, malnutrition are some factors to consider – and even death, though this is fortunately rare.
Meanwhile, sudden weight loss is likely to contribute to some excess skin and rolls over the body, which might require another surgery to remove.
Despite this, NYU Langone Health and NYC Health + Hospitals found that patients who had a sleeve gastrectomy or a gastric bypass lost an average of 58 pounds after two years, compared to an average of 12 pounds for patients on GLP-1 for six months.
The study recognises that the surgical group of patients had a longer time to lose the weight, however, the scientists claim those who opt for the injections struggle to keep the momentum going, finding 70 percent give up taking GLP-1 drugs within 12 months.
Of those who do commit to a full year on the jabs, patients in the study only lost seven percent of their body weight overall, compared to 24 percent for those who went under the knife two years before.
Featured Image Credit: Getty Images/Tatsiana Volkava
Topics: Ozempic, Health, World News, Food and Drink, Fitness
A study reveals potentially fatal side effects of GLP-1 drugs, that people are using to lose weight.
It’s well known that using GLP-1 drugs has proven to give out amazing weight loss results to many, but while beneficial to people who want to lose weight, the side effects vary greatly by severity.
From tooth decay to a saggy butt and hair loss, or an increased risk of thyroid cancer – sometimes it’s just not worth it.
There are also the more common gastrointestinal issues, which have led to some people cutting the drug out of their lives forever.
However, now a UK regulator has come out to expose potential deadly side effects of using the drugs.
The Medicines and Healthcare products Regulatory Agency’s Yellow Card scheme is the UK’s system for collecting reports of suspected side effects or negatives reactions to drugs.
Concerning GLP-1’s, it has received nearly 400 reports of serious pancreas trouble from UK users.
The UK is opening up an investigation into GLP-1 and pancreatitis (Peter Dazeley / Contributor / Getty)
According to UK reports, there have been approximately 10 deaths linked to this condition thanks to the GLP‑1 drugs.
While pancreatitis is an uncommon reaction, the UK is still investigating it due to the risk of death.
As for who it can happen to, the MHRA told the Guardian: “Sometimes genes can influence the side‑effects an individual experiences when taking a medicine.
“Patient safety is Lilly’s top priority,” the company that makes Mounjaro told the Guardian in a statement.
A spokesperson told UNILAD that the MHRA is investigating the risk of acute pancreatitis (inflamed pancreas) from GLP-1 injections and whether or not it may be influenced by an individual’s genes.
Similarly, Novo Nordisk, which produces Ozempic and Wegovy, told the outlet: “We recommend that patients take these medications only for their approved indications and under the strict supervision of a healthcare professional, who can also advise on potential side effects.
“We continuously collect safety data on our marketed GLP-1 medicines and work closely with the authorities to ensure patient safety. The benefit-risk profile of our GLP-1 medicines remains positive, and we welcome any new research that will improve our understanding of treatments for people living with chronic diseases.”
A spokesperson for Lilly told UNILAD: “Patient safety is Lilly’s top priority. We take reports regarding patient safety seriously and actively monitor, evaluate, and report safety information for all our medicines. Adverse events should be reported under the MHRA’s Yellow Card scheme, but may be caused by other factors, including pre-existing conditions.”
GLP-1’s are said to cause (in some cases) acute pancreatitis which led to 10 deaths in the nation (UCG / Contributor / Getty)
A case study that is being studied regarding pancreatitis describes a 36-year-old woman in the US who ‘developed acute pancreatitis within five weeks of exposure to semaglutide therapy’.
The paper also made reference to a ‘study involving 1,269 hospitalized cases of acute pancreatitis demonstrated that patients who were exposed to GLP-1 RAs within the last 30 days were over twice as likely to develop pancreatitis’.
In 2024, data found that there had been 6,751 reports of acute pancreatitis associated with GLP-1 drugs between the years of 2005 to 2023.
According to a 2025 study on the link, ‘although these drugs are effective in improving blood sugar control and cardiovascular health, their association with pancreatitis remains an area of concern’.
However, a 2024 study which aimed to prove whether it did in fact cause more acute pancreatitis (AP) cases claimed that there was no such link for the study authors.
It said: “This real-world study did not observe a higher frequency of AP with GLP-1RA exposure in adults with T2D and a prior history of AP regardless of etiology.”
The results of the MHRA investigation will come out in due course to provide clarity on the situation.
UNILAD reached out to Lilly and Novo Nordisk for comment.
Featured Image Credit: Getty Images/Steve Christo – Corbis
A woman has explained just how much happier she is now that she has lost weight, but admits there was one serious side effect of the weight loss medication she used that she wasn’t expecting.
Weight loss drugs have quickly become all the rage, with many using them after being prescribed by a health professional.
Being praised by celebrities and normal citizens alike, many have found themselves wishing their doctor would prescribe a GLP-1 drug to help them shed the pounds.
While there are many stories of people loving the results, there have also been instances where the reported side effects have proven to be rather detrimental.
British woman Shona has explained her experience after getting on weight loss medication Mounjaro.
She noted that she has loved the way she looks after just being on it for five months; however, she’s also issued a warning to others.

A woman has spoken about the positives after getting on a weight loss drug (Getty Stock Image)
Writing in a Mail Online op-ed, she explained: “I’ve been on it since the beginning of February and have lost 17kg – the equivalent of around two pounds a week.
“While I was 78kg (12st 4lb) when I started, I’m now 61kg (9st 10lb) and in this respect I couldn’t be more delighted. I haven’t been this weight since before the birth of my fourth child, Dolly, in 2009.
“It has been 16 years of watching the scales ominously creeping upwards, during which time I have been through perimenopause and then menopause. And it has taken just five incredible months to lose it all again. I can’t explain how amazing that feels.”
She also highlighted the positive comments she has gotten from her friends and families. However, she says she feels like she has developed an eating disorder as a result of being on the weight loss drug.
Explaining that she made herself throw up after consuming a cinnamon pastry, noting that feelings of guilt kicked in.
She wrote: “The truth is unsettling and something I would never have predicted, but I can no longer ignore the darker side of what is happening to me.
“The fact is that using fat jabs has at the very least encouraged an unhealthy relationship with food, and at the very worst triggered an eating disorder. This is the Mounjaro mind game nobody talks about. I now see food as the enemy, something to be avoided at all cost.”

The woman has said she feels she has developed an unhealthy relationship with food as a result (Getty Stock Image)
Shona also quoted doctor Tom Hildebrandt, who said he was seeing an increase in patients with eating disorders taking weight-loss drugs.
She quoted him as saying: “In some cases, a person’s brain may interpret such dramatic, sudden weight loss as starvation, making people more obsessive about food.
“People who are taking these new weight-loss drugs may then find themselves compelled to further limit how much food they eat, even when it endangers their health.”
In response, the maker of Mounjaro, Eli Lilly, told UNILAD: “Patient safety is Lilly’s top priority, and we actively engage in monitoring, evaluating, and reporting safety information for all our medicines.
“Mounjaro (tirzepatide) should only be used when prescribed and supervised by a licensed healthcare professional, and prescriptions should be fulfilled and supplied by registered pharmacies or providers.
“We encourage patients to consult their doctor or other healthcare professional to discuss any possible side effects they may be experiencing.”
Featured Image Credit: Getty Stock Image

A new drug could help counter one of Wegovy’s most concerning side effects while also enhancing weight loss, a new study suggests.
Injectable weight-loss drugs have surged in popularity, largely driven by the rise of Ozempic, a diabetes medication that’s broken into pop culture thanks to its fast-working results.
Like any medication, though, Novo Nordisk’s Wegovy and Ozempic and Eli Lilly’s Mounjaro – which all contain a GLP-1 receptor agonist – carry side effects.
Some can be mild, from stomach aches and bloating to tiredness and feeling sick. But one worrying consequence of rapid weight loss can impact our health in the longer term: loss of muscle mass.
This can increase the risk of falls, decrease your overall strength and lead to sarcopenia – the gradual loss of muscle mass, strength and function.
Now, though, a new drug from US pharmacy Regeneron could, when taken alongside Wegovy, help preserve muscle mass while aiding with weight loss.

Weight loss drugs don’t usually come without their side effects (Mapo/Getty Images)
A study, which is still only in its mid-stages, found the drug, trevogrumab, helped patients preserve up to 51 percent of lean mass, reports Reuters.
Some 599 patients were involved, and it was found that people who took just Wegovy lost fat, but also lost about 7.9 pounds of muscle.
People who took a combination of Wegovy and trevogrumab lost less muscle – 3.7 pounds – and more fat.
The pairing helped people lose up to 11.3 percent of their body weight, compared to 10.4 percent with Wegovy alone.
If future data holds up, trevogrumab could become the first add-on therapy to fix a major side effect of GLP-1s, making treatments like Wegovy even more appealing and, more importantly, better tolerated.

US pharmaceutical company Regeneron are behind the new trial, in its mid-stages (Cheng Xin/Getty Images)
The US Food and Drug Administration said drugs need to show ‘additional weight loss or other benefits,’ such as improvement in patients’ strength, for them to be considered for approval, however.
More data is needed on trevogrumab’s safety, as well as its long-term effects on strength and overall health.
A triple drug combination was also tested – of Wegovy, Regeneron’s trevogrumab and another antibody garetosmab.
While it helped patients lose 13.2 percent of their body weight, it also yielded the highest rate of side effects among all the groups studied – 28 percent of people stopped taking the drug.
Two people in the triple combination group also died, but Regeneron does not yet know if this is linked to their treatment.
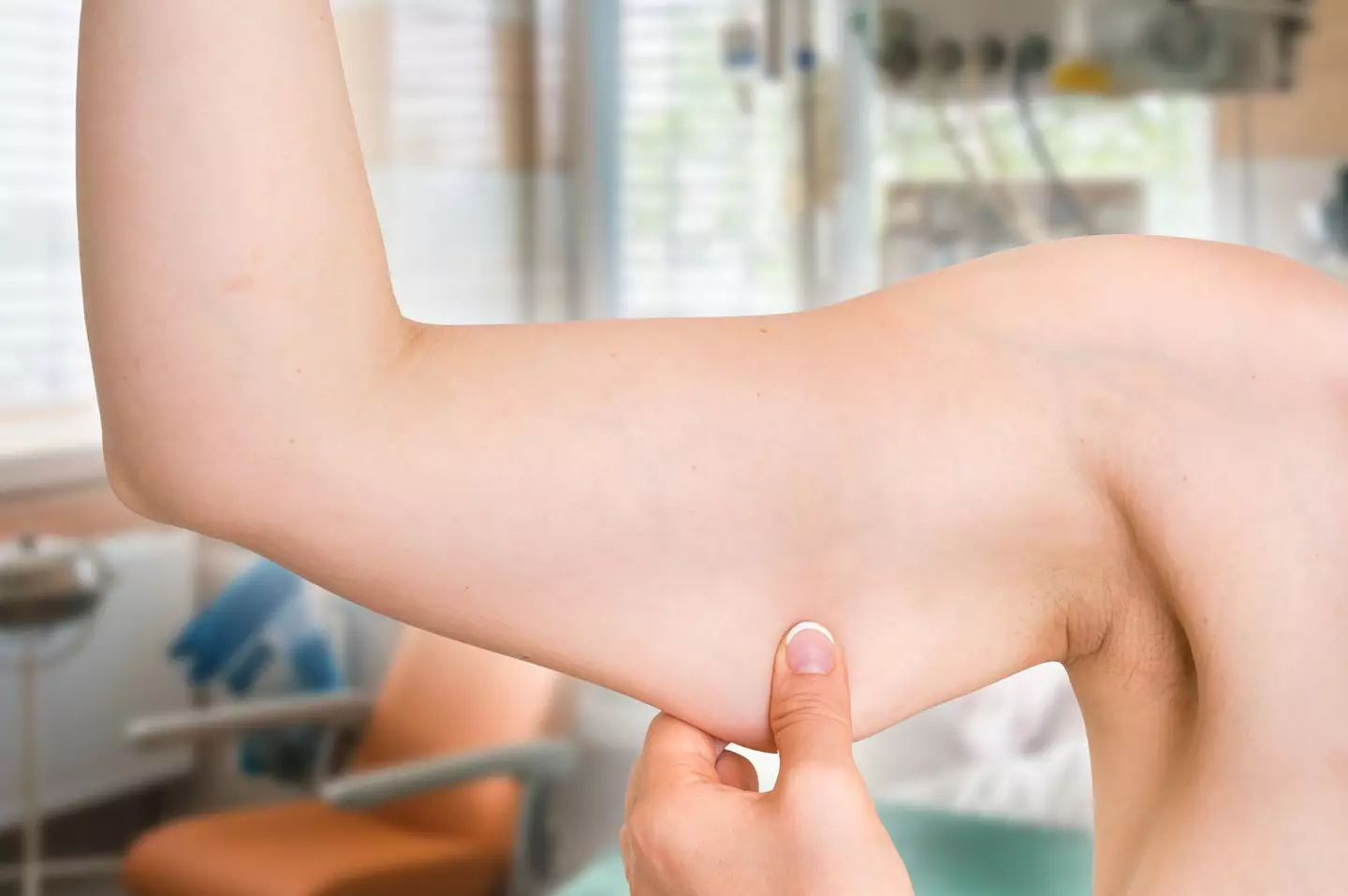
Losing muscle mass can have serious impacts on our long-term health (Andriano_cz/Getty Images)
UNILAD has contacted Regeneron for comment.
A Novo Nordisk spokesperson told UNILAD, in part: “Currently available data on body composition do not indicate an association of greater lean body mass loss over fat mass loss with semaglutide treatment and neither has any safety signal been identified in the clinical and post marketing data in relation to lean mass loss with semaglutide.
“Novo Nordisk continues to collect safety data on our marketed GLP-1 RAs and collaborate closely with the authorities to ensure patient safety.”
Featured Image Credit: Tatsiana Volkava/Getty Images